Abstract
Anti-CD19 chimeric antigen receptor (CAR) T cells can eliminate lymphoma, but CD19 loss from lymphoma can cause anti-CD19 CAR T-cell treatment failure. To address CD19 loss, we designed a bicistronic construct encoding 2 CARs. The first CAR in the bicistronic construct was the anti-CD19 CAR Hu19-CD828Z, which incorporated a human anti-CD19 single-chain variable fragment (scFv) along with CD28 and CD3ζ domains (Brudno et al. Nature Medicine 2020). The 2nd CAR in the construct was a novel anti-CD20 CAR incorporating a human anti-CD20 scFv, plus 4-1BB and CD3ζ domains. This bicistronic CAR construct, designated Hu1928-Hu20BB-original, was encoded by the mouse stem cell virus-based splice-gag (MSGV1) gamma-retroviral vector.
Repeated segments of identical DNA sequence can lead to recombination events when retroviral vectors are utilized. Recombination events driven by repeated identical nucleotide segments can occur during retroviral vector production in packaging cells. Recombination events can also occur in transduced T cells during reverse transcription when repeated segments of identical nucleotides on the same nucleotide strand anneal, which leads to strand switching by the reverse transcriptase enzyme and deletion of part of the sequence.
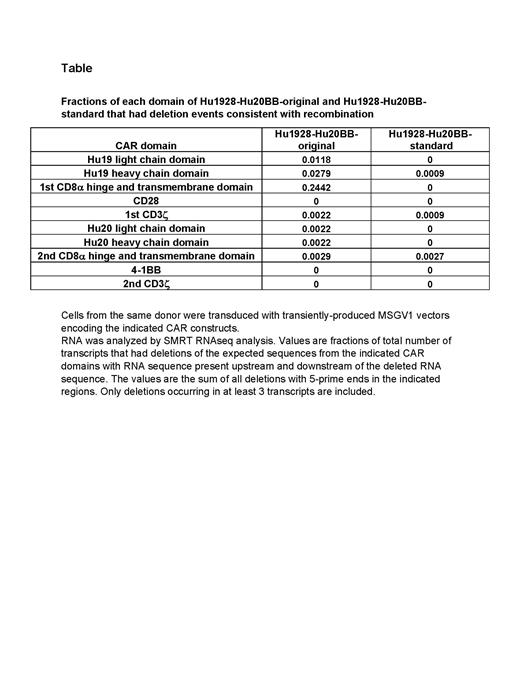
We assessed T cells transduced with Hu1928-Hu20BB-original for evidence of recombination. Southern blots of DNA from T cells transduced with Hu1928-Hu20BB-original showed a dominant band of the expected CAR transgene length and no aberrant bands after 21 hours of exposure; however, with extended exposure of 8 days, an unexpected band indicating shorter transgene length became visible. This aberrant band was consistent with deletion of part of the transgene sequence due to recombination. To investigate possible recombination by a more sensitive method, we assessed RNA from transduced T cells by single-molecule real-time RNA sequencing (SMRT-RNAseq, PacBio). Deletions of Hu1928-Hu20BB-original RNA sequence between segments of identical sequence were detected by SMRT-RNAseq. These findings were consistent with recombination. The most common recombination events were between the CD8α hinge and transmembrane (HT) domains of the 2 CARs of Hu1928-Hu20BB-original. These HT domains shared regions of identical nucleic acid sequence. Less commonly, recombination events occurred between segments of identical sequence shared by the heavy-chain domains and the light-chain domains of the two CARs in the construct. The attached table summarizes deletions consistent with recombination in Hu1928-Hu20BB-original RNA from transduced T cells of one patient. Similar results were obtained in T cells of 3 other patients. We confirmed deletions consistent with recombination in CAR RNA transcripts by short-read RNA sequencing (Illumina). Digital droplet PCR analysis of DNA from transduced T cells showed that recombination events between the HT domains of Hu1928-Hu20BB-original were present in DNA of transduced T cells.
We modified the DNA sequence of Hu1928-Hu20BB-original to reduce repeated segments of identical DNA sequence to form a new CAR designated Hu1928-Hu20BB-standard. When T cells transduced with Hu1928-Hu20BB-standard were studied by SMRT RNAseq, deletions of RNA sequence consistent with recombination were substantially reduced (Table).
We modified Hu1928-Hu20BB-standard by lengthening the linker in the anti-CD20 scFv to form a construct designated Hu1928-Hu20BB-long. We compared Hu1928-Hu20BB-standard to Hu1928-Hu20BB-long in vitro, and both specifically recognized CD19 and CD20 in assays of degranulation, cytokine release, and proliferation. In vitro assays showed higher interleukin-2 release by T cells transduced with Hu1928-Hu20BB-long versus Hu1928-Hu20BB-standard in response to 3 different CD20+ target cells (P value range 0.001 to 0.036). We tested Hu1928-Hu20BB-long against tumors established in nod-scid common gamma-chain knockout (NSG) mice. Human T cells expressing Hu1928-Hu20BB-long eliminated tumors of the following tumor cell lines: st486 (CD19+, CD20+), st486-CD19-negative (CD20+, CD19 low to negative expression), and NALM6 (CD19+, CD20 low expression). One million Hu1928-Hu20BB-long T cells per mouse eradicated st486 tumors. These optimized anti-CD19/CD20 bicistronic CAR constructs are promising for clinical treatment of B-cell malignancies.
Lam: Kite, a Gilead Company: Patents & Royalties. Kochenderfer: Bristol Myers Squibb: Research Funding; Kite, a Gilead Company: Patents & Royalties: on anti-CD19 CARs, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal